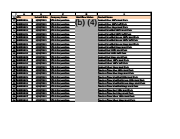
Date of Substantial Equivalence submissions to FDA
| Tracking # |
2015-8471 |
| Submitted | Oct. 19, 2015 |
MuckRock users can file, duplicate, track, and share public records requests like this one. Learn more.
Communications
From: ben zeidler
To Whom It May Concern:
This is a request under the Freedom of Information Act. I hereby request the following records:
Please provide the dates that KT&G Corporation submitted Substantial Equivalence reports for each of its products by brand and subbrand.
Timeless Time
Red 84 King Box
Red 100 Box
Blue 84 King Box
Blue 100 Box
Silver 84 King Box
Silver 100 Box
Menthol 84 King Box
Menthol 100 Box
Menthol Green 84 King Box
Menthol Green 100 Box
Carnival
Red 100 Soft
Red 100 Box
Red King Box
Blue 100 Soft
Blue 100 Box
Blue King Box
Silver 100 Soft
Silver 100 Box
Silver King Box
Menthol 100 Soft
Menthol 100 Box
Menthol King Box
Menthol Green 100 Soft
Menthol Green 100 Box
Menthol Green King Box
The requested documents will be made available to the general public, and this request is not being made for commercial purposes.
In the event that fees cannot be waived, I would be grateful if you would inform me of the total charges in advance of fulfilling my request. I would prefer the request filled electronically, by e-mail attachment if available or CD-ROM if not.
Thank you in advance for your anticipated cooperation in this matter. I look forward to receiving your response to this request within 20 business days, as the statute requires.
Sincerely,
Ben Zeidler
From: Food and Drug Administration
An acknowledgement letter, stating the request is being processed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: MuckRock.com
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information request, copied below, and originally submitted on Oct. 19, 2015. Please let me know when I can expect to receive a response, or if further clarification is needed. You had assigned it reference number #2015-8471.
Thanks for your help, and let me know if further clarification is needed.
From: Russ, Wilson
Good morning,
Your FOIA Request 2015-8471 has been completed and closed. The records were sent around 9/20/2016 to the address that was given on the faxed request.
Wilson M. Russ
Program Support Specialist
Office of the Executive Secretariat
Division of Freedom of Information
U.S. Food and Drug Administration
Tel: 301-796-8981
wilson.russ@fda.hhs.gov<mailto:wilson.russ@fda.hhs.gov>
[cid:image001.png@01D1C57E.DFA022A0]<http://www.fda.gov/>
[cid:image002.jpg@01D1C57E.DFA022A0]<https://www.facebook.com/FDA> [cid:image003.jpg@01D1C57E.DFA022A0] <https://twitter.com/US_FDA> [cid:image004.jpg@01D1C57E.DFA022A0] <http://www.youtube.com/user/USFoodandDrugAdmin> [cid:image005.jpg@01D1C57E.DFA022A0] <http://www.flickr.com/photos/fdaphotos/> [cid:image006.jpg@01D1C57E.DFA022A0] <http://www.fda.gov/AboutFDA/ContactFDA/StayInformed/RSSFeeds/default.htm>
From: ben zeidler
Those records were never received in this office, could you please resend? Thank you.
From: Russ, Wilson
Good morning,
Attached are the records responsive to your request. If you have any questions, please feel free to contact me.
Thank you,
Wilson M. Russ
Program Support Specialist
Office of the Executive Secretariat
Division of Freedom of Information
U.S. Food and Drug Administration
Tel: 301-796-8981
wilson.russ@fda.hhs.gov<mailto:wilson.russ@fda.hhs.gov>
[cid:image001.png@01D1C57E.DFA022A0]<http://www.fda.gov/>
[cid:image002.jpg@01D1C57E.DFA022A0]<https://www.facebook.com/FDA> [cid:image003.jpg@01D1C57E.DFA022A0] <https://twitter.com/US_FDA> [cid:image004.jpg@01D1C57E.DFA022A0] <http://www.youtube.com/user/USFoodandDrugAdmin> [cid:image005.jpg@01D1C57E.DFA022A0] <http://www.flickr.com/photos/fdaphotos/> [cid:image006.jpg@01D1C57E.DFA022A0] <http://www.fda.gov/AboutFDA/ContactFDA/StayInformed/RSSFeeds/default.htm>
Files
pages