Alberto Geddis Martinez, AKA "Alpo" (June 8, 1966 – October 31, 2021)
It is a clone of this request.
| Tracking # |
22-00092-F |
| Submitted | Nov. 1, 2021 |
MuckRock users can file, duplicate, track, and share public records requests like this one. Learn more.
Communications
From: T. McElwee
To Whom It May Concern:
Pursuant to the Freedom of Information Act, I hereby request the following records:
The results of a search of the NADDIS pointer index for the Investigative Reporting and Filing System, for the name and identifying information of Alberto Geddis Martinez, a known drug trafficker.
Martinez's death has been widely reported (see attachment in PDF) and privacy concerns recognized under FOIA and Privacy Act are diminished post-mortem. Records are releasable in the public interest, due to Martinez's notoriety as a high-level dealer as part of a known Drug Trafficking Organization.
To identify records, the following information is known about Martinez:
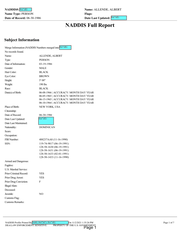
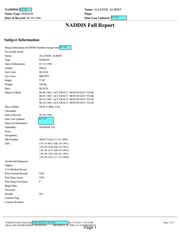
Full name: Alberto Geddis Martinez
Nickname: Alpo
Known Alias: Abraham Rodriguez
Date of Birth: June 8, 1966
Date of Death: October 31, 2021
The requested documents will be made available to the general public, and this request is not being made for commercial purposes.
In the event that there are fees, I would be grateful if you would inform me of the total charges in advance of fulfilling my request. I would prefer the request filled electronically, by e-mail attachment if available or CD-ROM if not.
Thank you in advance for your anticipated cooperation in this matter. I look forward to receiving your response to this request within 20 business days, as the statute requires.
Sincerely,
T. McElwee
From: Drug Enforcement Administration
Dear Mr. McElwee: This is to acknowledge receipt of your Freedom of Information Act/Privacy Act (FOIA/PA) request by the Drug Enforcement Administration, FOIA/PA Section. This response is a courtesy reply. It does not replace the acknowledgement letter that will be sent to you once your request has been reviewed by our Intake Unit. Please note, requests are handled in the order they are received. We will advise you if any additional information is required. Please be advised, due to necessary operational changes as a result of the national emergency concerning the novel coronavirus disease (COVID-19) outbreak, there may be some delay in the processing of your request. You may wish to visit DEA’s public facing website at or the FOIA Library available (http://www.dea.gov/) www.dea.gov at FOIA Library | DEA.gov to determine if the information you seek is already available to the public. You may contact our FOIA Public Liaison at (571) 776-3139 for any further assistance and to discuss any aspect of your request. Additionally, you may contact the Office of Government Information Services (OGIS) at the National Archives and Records Administration to inquire about the FOIA mediation services they offer. The contact information for OGIS is as follows: Office of Government Information Services, National Archives and Records Administration, Room 2510, 8601 Adelphi Road, College Park, Maryland 20740-6001; e-mail at ogis@nara.gov; telephone at (202) 741-5770; toll free at 1-877-684-6448; or facsimile at (202) 741-5769. If you have any questions regarding this email, you may contact our FOIA Requester Service Center at (571) 776-2300. Thank you for your patience as we work to complete the processing of your request. Sincerely, Jennifer Chavez FOIA/PA Specialist
Intake Unit FOIA/PA Section Drug Enforcement Administration Telephone: (571) 776-3261 E-mail: Jennifer.M.CoreasChavez@dea.gov PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal andprivileged information that requires protection in compliance with the PrivacyAct of 1974 (5 U.S.C. § 552a). Any useof this information by anyone other than the intended recipient is prohibited. If you have received this communication inerror, please immediately delete all copies of this information and notify meby e-mail. The use, dissemination,distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful.
From: Drug Enforcement Administration
Dear Mr. McElwee: Please see the attached response to your Freedom of Information/Privacy Act request 22-00092-F. If you have any questions, please contact our FOIA Requester Service Center at (571) 776-2300. Sincerely, Jennifer Coreas-Chavez FOIA Specialist (Intake FSRN Unit)
Freedom of Information/Privacy Act Section Drug Enforcement Administration Telephone: 571-776-3261 PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal andprivileged information that requires protection in compliance with the PrivacyAct of 1974 (5 U.S.C. § 552a). Any useof this information by anyone other than the intended recipient is prohibited. If you have received this communication inerror, please immediately delete all copies of this information and notify meby e-mail. The use, dissemination,distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful.
From: Muckrock Staff
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information Act request, copied below, and originally submitted on Nov. 1, 2021. Please let me know when I can expect to receive a response. You had assigned it reference number #22-00092-F.
Thanks for your help, and let me know if further clarification is needed.
From: Drug Enforcement Administration
Good Morning,
Please see the attached status to your Freedom of Information/Privacy Act request 22-00092-F. If you have any questions, please contact me at the phone number or e-mail address provided in the attached letter.
Thanks,
Denise M. Gutrick
Government Information Specialist
Processing Sub-Unit (CCARP)
FOIA and Privacy Act Unit
Drug Enforcement Administration (DEA)
Tel: (571) 776-2913
Email: Denise.M.Gutrick@dea.gov<mailto:Denise.M.Gutrick@dea.gov>
PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal and privileged information that requires protection in compliance with the Privacy Act of 1974 (5 U.S.C. § 552a). Any use of this information by anyone other than the intended recipient is prohibited. If you have received this communication in error, please immediately delete all copies of this information and notify me by e-mail. The use, dissemination, distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful.
From: Muckrock Staff
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information Act request, copied below, and originally submitted on Nov. 1, 2021. Please let me know when I can expect to receive a response. You had assigned it reference number #22-00092-F.
Thanks for your help, and let me know if further clarification is needed.
From: Muckrock Staff
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information Act request, copied below, and originally submitted on Nov. 1, 2021. Please let me know when I can expect to receive a response. You had assigned it reference number #22-00092-F.
Thanks for your help, and let me know if further clarification is needed.
From: Drug Enforcement Administration
Good Morning,
This email is to notify you that you will be receiving a Secure Message:
In an effort to better protect information that the Department of Justice (DOJ) maintains about individuals, we have implemented a tool to prevent accidental disclosures of sensitive personally identifiable information (PII). Documents that are responsive to a FOIA or Privacy Act request may contain sensitive PII. To safeguard this information, any email containing sensitive PII will be encrypted. Accordingly, you may receive an alert that that an encrypted message has been delivered to you. Should you receive such an alert, please use the attached instruction to decrypt and save the message.
Thanks,
Denise M. Gutrick
Government Information Specialist
Processing Sub-Unit (CCARP)
FOIA and Privacy Act Unit
Drug Enforcement Administration (DEA)
Tel: (571) 776-2913
Email: Denise.M.Gutrick@dea.gov<mailto:Denise.M.Gutrick@dea.gov>
PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal and privileged information that requires protection in compliance with the Privacy Act of 1974 (5 U.S.C. § 552a). Any use of this information by anyone other than the intended recipient is prohibited. If you have received this communication in error, please immediately delete all copies of this information and notify me by e-mail. The use, dissemination, distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful.
From: Drug Enforcement Administration
This is a secure message. Click here https://secureemail.usdoj.gov/formpostdir/securereader?id=u5EqabZesBcr9mQiyEEcNMjK-VdSW-75&brand=1294dc92 by 2022-03-04 16:53 UTC to read your message. After that, open the attachment.
Good Morning,
Please see the attached response to your Freedom of Information/Privacy Act request 22-00092-F. If you have any questions, please contact me at the phone number or e-mail provided in the attached letter.
Thanks,
Denise M. Gutrick
Government Information Specialist
Processing Sub-Unit (CCARP)
FOIA and Privacy Act Unit
Drug Enforcement Administration (DEA)
Tel: (571) 776-2913
Email: Denise.M.Gutrick@dea.gov
PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal and privileged information that requires protection in compliance with the Privacy Act of 1974 (5 U.S.C. § 552a). Any use of this information by anyone other than the intended recipient is prohibited. If you have received this communication in error, please immediately delete all copies of this information and notify me by e-mail. The use, dissemination, distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful.
From: Drug Enforcement Administration
Dear T. McElwee: This e-mail responds to your Freedom of Information Act/Privacy Act (FOIA/PA) request dated May 18, 2022, received by the Drug Enforcement Administration (DEA), FOIA/PA Unit, seeking access to DEA records. Your request has been opened and assigned the following case number: 22-00780-F. Please include this case number when communicating with this office. The records you seek require searches in another office or offices, and so your request falls within "unusual circumstances." See 5 U.S.C. § 552(a)(6)(B)(i)-(iii). Because of these unusual circumstances, we are extending the time limit to respond to your request beyond the ten additional days provided by the statute. We have not yet completed a search to determine whether there are records within the scope of your request. The time needed to process your request will necessarily depend on the complexity of our records search and on the volume and complexity of any records located. For your information, this office assigns incoming requests to one of three tracks: simple, complex, or expedited. Each request is then handled on a first-in, first-out basis in relation to other requests in the same track. Simple requests usually receive a response in approximately one month, whereas complex requests necessarily take longer. At this time, your request has been assigned to the complex track. You may wish to narrow the scope of your request to limit the number of potentially responsive records or agree to an alternative time frame for processing, should records be located; or you may wish to await the completion of our records search to discuss either of these options. We regret the necessity of this delay, but please be assured that your request will be processed as soon as possible. If you have any questions or wish to discuss reformulation or an alternative time frame for the processing of your request, you may contact FOIA/PA Unit representative Sandra L. Seidel at (571) 776-3317 or via e-mail at Sandra.L.Seidel@dea.gov, our FOIA Requester Service Center at (571) 776-2300, or e-mail your correspondence to DEA.FOIA@dea.gov. In addition, you may wish to visit our website at www.dea.gov to determine if the information you are requesting is already available to the public. You may contact our FOIA Public Liaison at (571) 776-2300 for any further assistance and to discuss any aspect of your request. Additionally, you may contact the Office of Government Information Services (OGIS) at the National Archives and Records Administration to inquire about the FOIA mediation services they offer. The contact information for OGIS is as follows: Office of Government Information Services, National Archives and Records Administration, Room 2510, 8601 Adelphi Road, College Park, Maryland 20740-6001; e-mail at ogis@nara.gov; telephone at (202) 741-5770; toll free at 1-877-684-6448; or facsimile at (202) 741-5769. Sincerely,
Sandra L. Seidel FOIA/PA Specialist Intake Sub-Unit FOIA/PA Unit Drug Enforcement Administration Telephone: (571) 776-3317 E-mail: Sandra.L.Seidel@dea.gov
PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal and privileged information that requires protection in compliance with the Privacy Act of 1974 (5 U.S.C. § 552a). Any use of this information by anyone other than the intended recipient is prohibited. If you have received this communication in error, please immediately delete all copies of this information and notify me by e-mail. The use, dissemination, distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful.
From: Drug Enforcement Administration
Dear T. McElwee: This e-mail responds to your Freedom of Information Act/Privacy Act (FOIA/PA) request dated June 16, 2022, received by the Drug Enforcement Administration (DEA), FOIA/PA Unit, seeking access to DEA records. Your request has been opened and assigned the following case number: 22-00794-F. Please include this case number when communicating with this office. The records you seek require searches in another office or offices, and so your request falls within "unusual circumstances." See 5 U.S.C. § 552(a)(6)(B)(i)-(iii). Because of these unusual circumstances, we are extending the time limit to respond to your request beyond the ten additional days provided by the statute. We have not yet completed a search to determine whether there are records within the scope of your request. The time needed to process your request will necessarily depend on the complexity of our records search and on the volume and complexity of any records located. For your information, this office assigns incoming requests to one of three tracks: simple, complex, or expedited. Each request is then handled on a first-in, first-out basis in relation to other requests in the same track. Simple requests usually receive a response in approximately one month, whereas complex requests necessarily take longer. At this time, your request has been assigned to the complex track. You may wish to narrow the scope of your request to limit the number of potentially responsive records or agree to an alternative time frame for processing, should records be located; or you may wish to await the completion of our records search to discuss either of these options. We regret the necessity of this delay, but please be assured that your request will be processed as soon as possible. If you have any questions or wish to discuss reformulation or an alternative time frame for the processing of your request, you may contact FOIA/PA Unit representative Fantasia L. Hogan-Bassey-Graham at (571) 776-2210 or via e-mail at Fantasia.L.Hogan-Bassey-Graham@dea.gov, our FOIA Requester Service Center at (571) 776-2300, or e-mail your correspondence to DEA.FOIA@dea.gov. In addition, you may wish to visit our website at www.dea.gov to determine if the information you are requesting is already available to the public. You may contact our FOIA Public Liaison at (571) 776-2300 for any further assistance and to discuss any aspect of your request. Additionally, you may contact the Office of Government Information Services (OGIS) at the National Archives and Records Administration to inquire about the FOIA mediation services they offer. The contact information for OGIS is as follows: Office of Government Information Services, National Archives and Records Administration, Room 2510, 8601 Adelphi Road, College Park, Maryland 20740-6001; e-mail at ogis@nara.gov; telephone at (202) 741-5770; toll free at 1-877-684-6448; or facsimile at (202) 741-5769. Sincerely,
Fantasia L. Hogan-Bassey-Graham FOIA/PA Specialist Intake Sub-Unit FOIA/PA Unit Drug Enforcement Administration Telephone: (571) 776-2210 E-mail: Fantasia.L.Hogan-Bassey-Graham@dea.gov
PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal and privileged information that requires protection in compliance with the Privacy Act of 1974 (5 U.S.C. § 552a). Any use of this information by anyone other than the intended recipient is prohibited. If you have received this communication in error, please immediately delete all copies of this information and notify me by e-mail. The use, dissemination, distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful.
From: T. McElwee
Ms. Hogan-Bassey-Graham,
This may sound strange, but I believe you sent me a response at the wrong email address (though I am likely the intended recipient).
To sort this out, can you please tell me what the subject of FOIA request 22-00794-F was? Because it is not listed in the form-letter email I received.
Thank you,
T. McElwee
From: T. McElwee
Ms. Seidel,
This may sound strange, but I believe you sent me a response at the wrong email address (though I am likely the intended recipient).
To sort this out, can you please tell me what the subject of FOIA request 22-00780-F was? Because it is not listed in the form-letter email I received.
Thank you,
T. McElwee
From: Muckrock Staff
To Whom It May Concern:
I wanted to follow up on the following Freedom of Information Act request, copied below, and originally submitted on Nov. 1, 2021. Please let me know when I can expect to receive a response. You had assigned it reference number #22-00092-F.
Thanks for your help, and let me know if further clarification is needed.
From: Drug Enforcement Administration
Good Morning,
If you have any questions regarding 22-00092-F, please contact Denise Gutrick<mailto:Denise.M.Gutrick@dea.gov>, they were the processor for your case that is now closed.
Contact:
Denise.M.Gutrick@dea.gov<mailto:Denise.M.Gutrick@dea.gov>
571-776-2913
Kind regards,
Fantasia Hogan-Bassey-Graham
IBSS Corp. Paralegal II Contractor
DEA Intake Sub-Unit, Freedom of Information and Privacy Act Unit
U.S. Department of Justice
Drug Enforcement Administration
Attn: FOIA/PA Unit
8701 Morrissette Drive
Springfield, Virginia 22152
P: (571) 776-2210
E: fantasia.l.hogan-bassey-graham@dea.gov<mailto:fantasia.l.hogan-bassey-graham@dea.gov>
From: Drug Enforcement Administration
Good Morning:
This email is to notify you that you will be receiving a Secure Message:
In an effort to better protect information that the Department of Justice (DOJ) maintains about individuals, we have implemented a tool to prevent accidental disclosures of sensitive personally identifiable information (PII). Documents that are responsive to a FOIA or Privacy Act request may contain sensitive PII. To safeguard this information, any email containing sensitive PII will be encrypted. Accordingly, you may receive an alert that that an encrypted message has been delivered to you. Should you receive such an alert, please use the attached instruction to decrypt and save the message.
Regards,
Denise M. Gutrick
Government Information Specialist
Processing Sub-Unit (CCARP)
FOIA and Privacy Act Unit
Drug Enforcement Administration (DEA)
Tel: (571) 776-2913
Email: Denise.M.Gutrick@dea.gov<mailto:Denise.M.Gutrick@dea.gov>
PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal and privileged information that requires protection in compliance with the Privacy Act of 1974 (5 U.S.C. § 552a). Any use of this information by anyone other than the intended recipient is prohibited. If you have received this communication in error, please immediately delete all copies of this information and notify me by e-mail. The use, dissemination, distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful.
From: Drug Enforcement Administration
"Good Morning,
This is in response to your follow up request for Freedom of Information Act request 22-00092-F. Attached is a copy of our previous response to you regarding the aforementioned request. If you have any questions, please contact me at the phone number or e-mail provided in the attached letter.
Best regards,
Denise M. Gutrick
Government Information Specialist
Processing Sub-Unit (CCARP)
FOIA and Privacy Act Unit
Drug Enforcement Administration (DEA)
Tel: (571) 776-2913
Email: Denise.M.Gutrick@dea.gov
PRIVACY ACT NOTICE: This e-mail (including any attachments herein) may contain personal and privileged information that requires protection in compliance with the Privacy Act of 1974 (5 U.S.C. § 552a). Any use of this information by anyone other than the intended recipient is prohibited. If you have received this communication in error, please immediately delete all copies of this information and notify me by e-mail. The use, dissemination, distribution, or reproduction of this communication by unintended recipients is not authorized and may be unlawful."
Files
pages